| actinoallolides A - E |  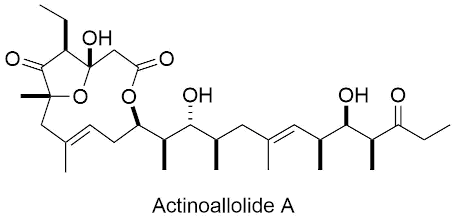 |
2019 |
Angew. Chem. Int. Ed. 2019, XX XXX-XXX. |
| aplyronines A and D |  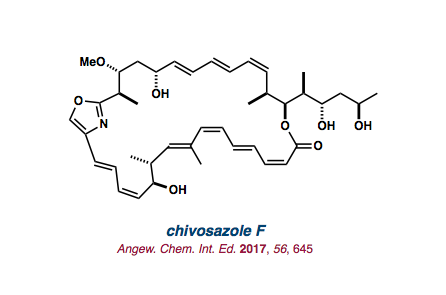 |
2018 |
Org. Biomol. Chem. 2018, 16 1343. |
| chivosazole F |   |
2017 |
Angew. Chem. Int. Ed. 2017, 56 645-649. |
| jiadifenolide |   |
2014 |
Angew. Chem. Int. Ed. 2014, 53 7286-7289. |
| (-)-leiodermatolide |  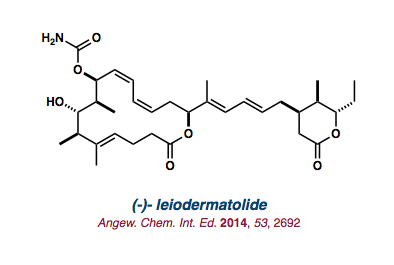 |
2014 |
Angew. Chem. Int. Ed. 2014, 53, 2692-2695. |
| (-)-rhizopodin |  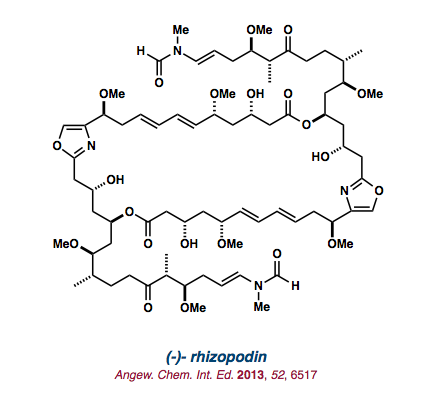 |
2013 |
Angew. Chem. Int. Ed. 2013, 52, 6517-6521. |
| aplyronine C |  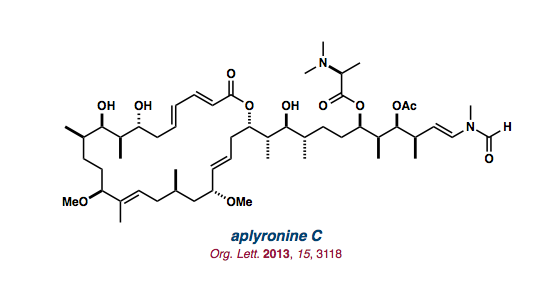 |
2013 |
Org. Lett. 2013, 15, 3118-3121 |
| alotaketal A |   |
2012 |
Org. Lett. 2012, 14, 5492-5495. |
| phorbaside A |  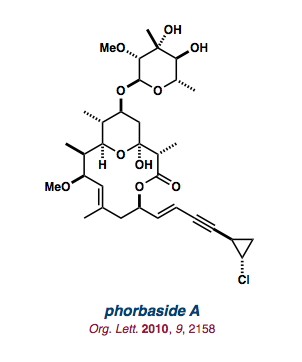 |
2010 |
Org. Lett. 2010, 12, 2158-2161. |
| spirangien A |   |
2009 |
Chem. Asian J. 2009, 4, 594-611. |
| (+)-neopeltolide |  |
2008 |
Chem. Commun. 2008, 37, 4708-4710. |
| (-)-saliniketals A & B |  |
2008 |
Org. Lett. 2008, 10, 3295-3298. |
| spirastrellolide A methyl ester |  |
2008 |
Angew. Chem. Int. Ed. 2008, 43, 3016-3020 & 3021-3025 |
| pteridic acids A & B |  |
2008 |
Tetrahedron 2008, 64, 4768-4777. |
| (-)-reidispongiolide A |
 | 2006 | Angew. Chem. Int. Ed. 2007, 6167-6171. |
| (+)-dolastatin 19 |
 | 2006 | Org. Lett. 2007, 2131-2134, Tetrahedron 2006, 5806-5819. |
| (-)-dictyostatin |  | 2004 | Angew. Chem. Int. Ed. 2004,
43, 4629-4633 |
| (-)-aurisides A and B | 
 | 2004 | Angew. Chem. Int. Ed. 2005,
44, 1130-1133 |
| (-)-callipeltoside A |  | 2003 | Org. Lett.
2003, 23, 4477-4480 |
| (+)-leucascandrolide A |  | 2002 | Angew. Chem. Int.
Ed. 2003, 42, 343-348. |
| siphonarin B |  | 2001 |
Org. Lett. 2002, 4, 391-394. |
| (+)-altohyrtin A/spongistatin 1 |  | 2001 | Angew. Chem. Int. Ed. 2001,
40, 4055-4060., Org. Biomol. Chem. 2005, 2399-2440. |
| (-)-laulimalide |  | 2001 |
Org. Lett. 2001, 3, 3149-3152. |
| (+)-discodermolide |  | 1999, 2001 |
Angew. Chem. 2000, 112, 385-388., J. Am.
Chem. Soc. 2001, 123, 9535-9544. |
| (-)-baconipyrone C |  | 1999 | Org. Lett.
2000, 2, 1513- 1516. |
| (+)-concanamycin F |  | 1999 | Angew. Chem. Int.
Ed. 2000, 39, 1308-1317. |
| (+)-elaiolide |  | 1999 |
Org. Lett. 1999, 1, 19-22. |
| (-)-tetrahydrolipstatin |  | 1999 | Tetrahedron Lett.
1999, 40, 393-394. |
| scytophycin C |  | 1997 | J.
Org. Chem. 1997, 62, 452-453. |
| zaragozic acid C intermediate |  | 1997 | Tetrahedron Lett.
1997, 38, 4301-4304. |
| (+)-restricticin |  | 1996 | Tetrahedron Lett. 1996,
37, 8243-8246. |
| (-)-ACRL toxin IIIB |  | 1994 | Tetrahedron Lett.
1994, 35, 9477-9480. |
| (-)-ebelactone A and B |  | 1993 | J. Org. Chem. 1995,
60, 3288-3300. |
| (-)-oleandolide |  | 1994 | J.
Am. Chem. Soc. 1994, 116, 11287-11314. |
| (-)-swinholide A |  | 1994 | J.
Am. Chem. Soc. 1994, 116, 9391-9392. |
| (-)-hemiswinholide A |  | 1994 | J. Am. Chem. Soc.
1994, 116, 9391-9392. |
| (+)-muamvatin |  | 1993 | J.
Am. Chem. Soc. 1993, 115, 1608-1610. |
| (-)-denticulatins A & B |  | 1992 | Tetrahedron Lett.
1992, 33, 801-804. |