Research
Research in the group ranges across the total synthesis of various biologically active natural products and structural analogues to the discovery and development of new synthetic methods, with particular interest in asymmetric reactions.
Stereocontrolled Synthesis of Bioactive Natural Products and Structural Analogues
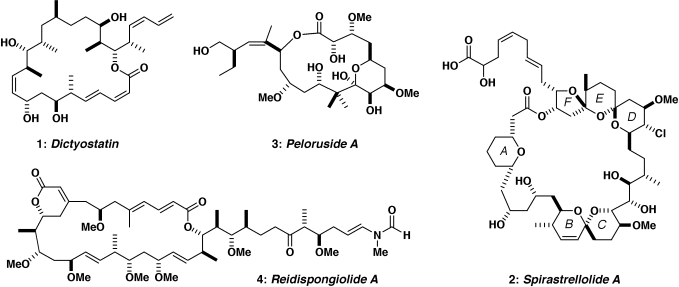
Current targets include rare anticancer agents of marine origin such as 1-4 below. For example, dictyostatin (1) has a promising antitumour profile and shares the same microtubule-stabilising mechanism as the clinically important drug Taxol, while spirastrellolide A (2) is a potent inhibitor of protein phosphatase 2A. Likewise, peloruside A (3) and reidispongiolide A (4) are novel antimitotic macrolides of marine origin, which also represent challenging synthetic targets. The initial uncertainty over the stereochemistry, combined with the lack of material from the natural source, has adversely affected their preclinical development. Efficient and flexible synthetic routes for the construction of these polyoxygenated structures are being developed to establish their full configurations and provide material for biological evaluation. A parallel objective is to design simplified analogues that retain the exceptional cancer cell growth inhibitory properties whilst increasing their synthetic accessibility.
New Synthetic Methods.
There is a need for new and more efficient methods of synthesis, particularly ones that achieve high levels of stereocontrol, where the development of asymmetric aldol methodology is of particular interest. These new methods are being applied to the synthesis of a wide variety of important natural products.
Selected Publications
- Total synthesis and configurational assignment of (-)-dictyostatin. Chem. Commun. 2004, 632; Angew. Chem. Int. Ed. 2004, 43, 4629
- Total synthesis of (+)-altohyrtin A / spongistatin 1 and structural analogues. Angew. Chem. Int. Ed. 2001, 40, 4055; Org. Biomol. Chem. 2005, 3, 2399, 2410, 2420, 2431, 2441.
- Total synthesis of (+)-discodermolide. J. Am. Chem. Soc. 2001, 123, 9535; J. Org. Chem. 2005, 70, 150.
- Synthetic studies towards reidispongiolide A. Proc. Natl. Acad. Sci. USA, 2004, 101, 11986.
