1. Synthesis of Bioactive Marine Metabolides.
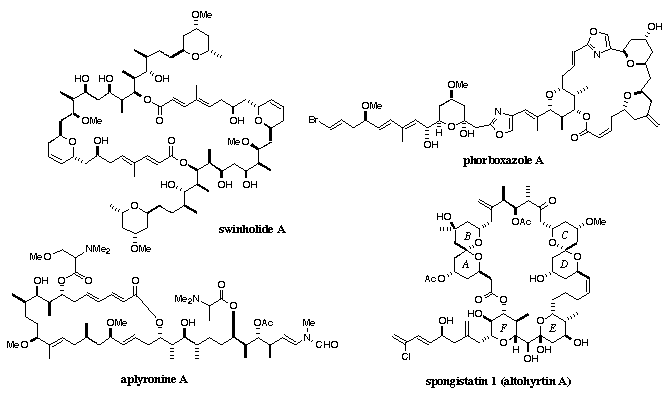
A large amount of effort in the group is currently focused on the synthesis of bioactive marine metabolites, with emphasis on novel macrocyclic compounds having antitumour properties. We have already completed the first total synthesis of swinholide A, a unique macrodiolide having a very large 44-membered ring. Swinholide A is a potent inhibitor of a range of human tumour cell lines, but is only available in microscopic quantities from the marine sponge Theonella swinhoei. A viable synthetic approach to the monomeric seco acid of swinholide A has been developed, followed by a bold dimerisation-macrolactonisation sequence without invoking selective protection. Swinholide A is one of the most complex marine natural products to have been synthesised to date. Current marine natural product targets include aplyronine A, spongistatin 1 (altohyrtin A), discodermolide, callipeltoside A, laulimalide, peloruside A, and the phorboxazoles.
This project area involves the development of new and practical methods for the construction of these complex target structures. Emphasis is placed on achieving a high level of control over the stereochemistry. The research programme has potential applications to the production of therapeutically useful compounds for cancer treatment. For example, spongistatin 1 (altohyrtin A) is among the most potent compounds tested against a distinctive subset of highly chemoresistant tumour types. While discodermolide and laulimalide share the same microtubule-stabilising mechanism as the anticancer drug Taxol™, they retain antimitotic potency against Taxol-resistant cancer cells. Due to their extremely scarce natural supply and the painstaking isolation procedure involved, synthetic efforts are urgently required to deliver further material for biological testing, as well as to provide novel analogues.
Selected publications
- Norcross, R. D.; Paterson, I. Total synthesis of bioactive marine macrolides. Chem. Rev. 1995, 95, 2041.
- Paterson, I.; Florence, G.; Gerlach, K.; Scott, J. P. Total synthesis of the antimicrotubule agent (+)-discodermolide using boron-mediated aldol reactions of chiral ketones. Angew. Chem. Int. Ed. 2000, 39, 377.
- Paterson, I.; Wallace, D. J.; Oballa, R. M. Studies in marine macrolide synthesis: synthesis of a fully functionalised C1-C28 subunit of spongistatin 1 (altohyrtin A). Tetrahedron Lett. 1998, 39, 8545.
- Paterson, I.; Woodrow, M. D.; Cowden, C. J. Studies in marine macrolide synthesis: construction of a 24-membered macrocyclic intermediate for aplyronine A. Tetrahedron Lett. 1998, 39, 6041. (e) Paterson, I.; Watson, C.; Yeung, K.-S.; Wallace, P. A.; Ward, R. A. Total synthesis of scytophycin C. J. Org. Chem. 1997, 62, 452.
- Paterson, I.; Yeung, K.-S.; Ward, R. A.; Cumming, J. G.; Smith, J. D. Total synthesis of swinholide A and hemiswinholide A. J. Am. Chem. Soc. 1994, 116, 9391.
Current Projects
- Synthesis of Bioactive Marine Metabolides.
- New Methods for the Synthesis of Polyketide Natural Products.
- Expanding Polyketide Diversity.
- New Reagents for Asymmetric Aldol Reactions.
- Biomimetic Total Synthesis of Polyether Ionophores.
- Designed Synthesis of Lophotoxin Analogues.
- Designed Synthesis of Novel Macrolide Antibiotics Related to Erythromycin.
- Transition State Modelling of Reactions (with Dr J. M. Goodman and Prof C. Gennari)
