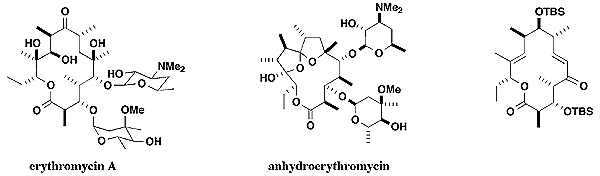
7. Designed Synthesis of Novel Macrolide Antibiotics Related to Erythromycin.
Erythromycin A is the most important member of the macrolide antibiotics of microbial origin. It is widely used in the treatment of many infectious diseases by oral administration and acts on the peptidyl transferase centre of the bacterial ribosome, so inhibiting protein synthesis. Antibiotic resistance is a growing clinical problem, leading to a recent resurgence of interest in the development of erythromycin derivatives having an enhanced spectrum of antibacterial potency to combat respiratory pathogens, including multi-resistant pneumococci. We are preparing novel libraries of erythromycin analogues as anti-infective agents by extension of our combinatorial methodology for solid phase polyketide synthesis. In contrast to conventional semi-synthetic efforts, variations in stereochemistry, substitution, and glycosidation can be approached in a completely systematic fashion and considerable structural diversification can be achieved.
Selected publications
- Paterson, I.; Rawson, D. J. Studies in macrolide synthesis: a highly stereoselective synthesis of (+)-(9S)-dihydroerythronolide A using macrocyclic stereocontrol. Tetrahedron Lett. 1989, 30, 7463.
- Paterson, I.; Laffan, D. D. P.; Rawson, D. J. Studies in macrolide synthesis: a concise asymmetric synthesis of a macrolide intermediate for the erythronolides. Tetrahedron Lett. 1988, 29, 1461.
Current Projects
- Synthesis of Bioactive Marine Metabolides.
- New Methods for the Synthesis of Polyketide Natural Products.
- Expanding Polyketide Diversity.
- New Reagents for Asymmetric Aldol Reactions.
- Biomimetic Total Synthesis of Polyether Ionophores.
- Designed Synthesis of Lophotoxin Analogues.
- Designed Synthesis of Novel Macrolide Antibiotics Related to Erythromycin.
- Transition State Modelling of Reactions (with Dr J. M. Goodman and Prof C. Gennari)
