4. New Reagents for Asymmetric Aldol Reactions.
The development of general and experimentally straightforward procedures for the asymmetric synthesis of β-hydroxyketones by aldol reactions of ketones with aldehydes is of continuing interest. These aldol products are immensely valuable in the total synthesis of complex polyoxygenated compounds. We have introduced a range of chiral ketones, which undergo highly diastereoselective syn and anti aldol reactions with aldehydes using kinetically generated boron enolates. In certain cases, 1,4-, 1,5- and even 1,6-stereoinduction is possible, enabling the control of remote stereocentres in acyclic systems. Moreover, asymmetric ethyl and methyl ketone aldol additions can be performed using chiral boron reagents (Ipc2BX, X = Cl or OTf) prepared from (+)- or (-)-α-pinene. These reagents control the relative and absolute stereochemistry of C—C bond formation between achiral and chiral ketones and aldehydes, thus providing a powerful new synthetic method.
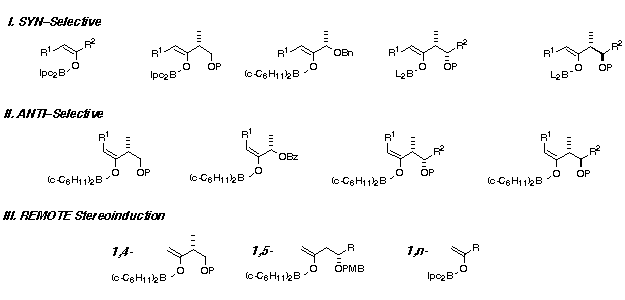
Selected publications:
- Cowden, C. J.; Paterson, I. Asymmetric aldol reactions using boron enolates. Org. React. 1997, 51, 1.
- Paterson, I.; Wallace, D. J.; Cowden, C. J. Polyketide synthesis using the boron-mediated, anti aldol reactions of lactate-derived ketones: total synthesis of (—)-ACRL Toxin IIIB. Synthesis 1998, 639.
- Paterson, I.; Gibson. K. R.; Oballa, R. M. Remote, 1,5-anti stereoinduction in the boron-mediated aldol reactions of β-oxygenated methyl ketones. Tetrahedron Lett. 1996, 37, 8585.
- Paterson, I.; Wallace, D. J.; Velazquez, S. M. Studies in polypropionate synthesis: high π-face selectivity in syn and anti aldol reactions of chiral boron enolates of lactate-derived ketones. Tetrahedron Lett. 1994, 35, 9083.
- Paterson, I.; Wallace, D. J. Manipulation of the aldol adducts from lactate-derived ketones. A versatile chiral auxiliary for the asymmetric synthesis of β-hydroxy carbonyl compounds. Tetrahedron Lett. 1994, 35, 9087.
- Paterson, I.; Tillyer, R. D. Studies in polypropionate synthesis: high π-face selectivity in syn aldol reactions of Sn(II) enolates from (R)- and (S)-1-(benzyloxy)-2-methylpentan-3-one. Tetrahedron Lett. 1992, 33, 4233.
- Paterson, I.; Goodman, J. M.; Lister, M. A.; Schumann, R. C.; McClure, C. K.; Norcross, R. D. Enantio- and diastereoselective aldol reactions of achiral ethyl and methyl ketones with aldehydes: the use of enol diisopinocampheyl-borinates. Tetrahedron 1990, 46, 4663.
Current Projects
- Synthesis of Bioactive Marine Metabolides.
- New Methods for the Synthesis of Polyketide Natural Products.
- Expanding Polyketide Diversity.
- New Reagents for Asymmetric Aldol Reactions.
- Biomimetic Total Synthesis of Polyether Ionophores.
- Designed Synthesis of Lophotoxin Analogues.
- Designed Synthesis of Novel Macrolide Antibiotics Related to Erythromycin.
- Transition State Modelling of Reactions (with Dr J. M. Goodman and Prof C. Gennari)
