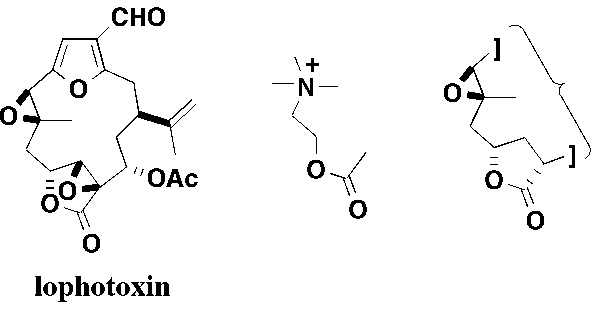
6. Designed Synthesis of Lophotoxin Analogues.
Lophotoxin, isolated from several species of the Pacific sea whip Lophogorgia, is a potent neurotoxin that irreversibly blocks nicotinic acetylcholinergic neurotransmission in autonomic ganglia causing paralysis and asphyxiation. Together with structural analogues, it offers considerable potential as a selective neuropharmacological probe for characterising the nicotinic acetylcholine receptor involved in synaptic transmission. Recent SAR work suggests that the pharmacophore required for irreversible inhibition of the receptor retains the γ-lactone and the trisubstituted epoxide, which may mimic the acetate oxygens and cationic ammonium group in acetylcholine. The design and synthesis of simplified structural analogues of lophotoxin with similar functionality and local conformation (as predicted by molecular modelling), which retain the potential function to act as potent ligands for the nicotinic acetylcholine receptor, is being investigated.
Selected publications
- Paterson, I.; Brown, R. E.; Urch, C. Studies towards the synthesis of lophotoxin and pukalide: synthesis of the 14-membered macrocyclic core and some acyclic structural analogues. Tetrahedron Lett. 1999, 40, 5807.
- Paterson, I.; Gardner, M.; Banks, B. J. Studies in marine cembranolide synthesis: a synthesis of 2,3,5-trisubstituted furan intermediates for lophotoxin and pukalide. Tetrahedron 1989, 45, 5283.
Current Projects
- Synthesis of Bioactive Marine Metabolides.
- New Methods for the Synthesis of Polyketide Natural Products.
- Expanding Polyketide Diversity.
- New Reagents for Asymmetric Aldol Reactions.
- Biomimetic Total Synthesis of Polyether Ionophores.
- Designed Synthesis of Lophotoxin Analogues.
- Designed Synthesis of Novel Macrolide Antibiotics Related to Erythromycin.
- Transition State Modelling of Reactions (with Dr J. M. Goodman and Prof C. Gennari)
