3. Expanding Polyketide Diversity.
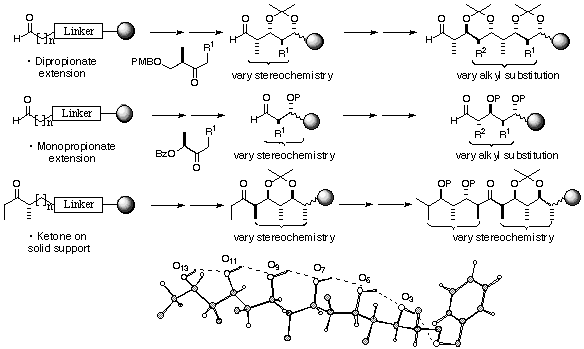
The polyketides represent a rich reservoir of structurally complex, bioactive natural products, with many having therapeutic importance. As a source of pharmaceutically relevant, molecular diversity, they are attractive targets for developing a combinatorial approach to library generation. To expand polyketide diversity, we are using a variety of chain extending units to achieve structural and stereochemical diversification, initially exploiting iterative aldol chemistry using readily accessible chiral ketone reagents developed in our laboratory. This research is generating novel classes of polyketide-type structures with multiple stereocentres, which are being synthesised in an efficient manner by chain extension of a solid-supported aldehyde or ketone. Notably, we have demonstrated unequivocally that the solid phase yields for multi-step reaction sequences are markedly better than the solution phase equivalent and high levels of diastereoselectivity are maintained. Ultimately, the assembly of libraries of diverse unnatural polyketides through variation in the building blocks, the number of chain extensions, the stereochemical information, oxidation state, and the introduction of unsaturation or rings should be feasible. Our long-term objectives in this area include the stereocontrolled synthesis of diverse polyketide sequences of comparable complexity to erythromycin and discodermolide. This represents some of the most sophisticated chemistry ever to have been performed on solid support.
The biological activity of polyketides is generally dependent on specific non-covalent interactions, where conformational preorganisation not only directs functional groups in space for target recognition but reduces the entropic penalty involved in binding. Our synthetic 1,3-polyol systems are designed to have distinct conformational preferences, which depend on the interplay of specific intramolecular hydrogen bonding patterns and the avoidance of syn-pentane steric interactions along the otherwise flexible hydrocarbon backbone.
Selected publications
- Paterson, I.; Donghi, M.; Gerlach, K. A combinatorial approach to polyketide-type libraries via iterative asymmetric aldol reactions performed on solid support. Angew. Chem. Int. Ed. 2000, 39, 3315.
- Paterson, I.; Scott, J. P. Laboratory emulation of polyketide biosynthesis: an iterative, aldol-based, synthetic entry to polyketide libraries using (R)- and (S)-1-(benzyloxy)-2-methylpentan-3-one, and conformational aspects of extended polypropionates. J. Chem. Soc., Perkin Trans. 1 1999, 1003.
- Paterson, I.; Scott. J. P. Polyketide library synthesis: iterative assembly of extended polypropionates using (R)- and (S)-1-(benzyloxy)-2-methylpentan-3-one. Tetrahedron Lett. 1997, 38, 7441.
Current Projects
- Synthesis of Bioactive Marine Metabolides.
- New Methods for the Synthesis of Polyketide Natural Products.
- Expanding Polyketide Diversity.
- New Reagents for Asymmetric Aldol Reactions.
- Biomimetic Total Synthesis of Polyether Ionophores.
- Designed Synthesis of Lophotoxin Analogues.
- Designed Synthesis of Novel Macrolide Antibiotics Related to Erythromycin.
- Transition State Modelling of Reactions (with Dr J. M. Goodman and Prof C. Gennari)
